Introduction: Bortezomib and daratumumab revolutionized the therapy for AL amyloidosis (AL). However, relapsed/refractory AL resistant to both drugs is an unmet clinical need, with limited options due to the frailty of these patients and paucity of prospective data. The safety profile and efficacy of belantamab mafodotin (BLM) in multiple myeloma (MM) are well known. In contrast, published data for AL are currently limited to case series of 6 (Zhang, Ann Hematol. 2022) and 12 patients only (Khwaga, Blood Cancer J. 2022). Here, we report a multicenter real-world data on 12 AL patients treated with BLM and compare these to the prior reports.
Methods: We retrospectively identified all AL amyloidosis patients who received BLM outside a clinical trial, between Feb 2020-Sep 2022. The diagnosis, staging, hematologic and organ responses and progression of AL were evaluated according to consensus criteria. Event-free survival (EFS) was defined as the time from the first day of BLM administration to hematologic progression or therapy change for inadequate response or death.
Results: Twelve AL patients from 5 centers in Israel and Italy were included. The median age at BLM initiation was 66 y (range 54-70). The most common FISH aberrations were 1q gain in 8 patients (67%) and t(11;14) in 5 (42%). All patients except one had cardiac involvement (92%) and 9 (75%) had renal involvement. The median number of prior lines of therapy was 3 (range 2-9). All patients previously received bortezomib, an alkylator and daratumumab, 11 (92%) received lenalidomide, 10 (83%) received pomalidomide and 9 (75%) underwent prior autologous transplant. Seven patients (58%) had Eastern cooperative oncology group performance status (ECOG-PS) of at least 2 at BLM initiation and half of the patients had AL Mayo stage of at least 3a.
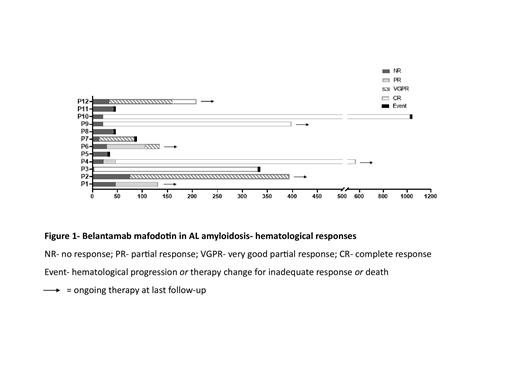
Most patients (10/12) received BLM as a single agent at 2.5mg/kg q3 weeks. The overall hematological response rate (ORR) to BLM was 75% (9/12), including 5 patients with complete response, 3 with very good partial response, and 1 with partial response (Figure 1).
Detailed biomarker-based evaluation of organ responses was missing in many patients, yet, of 4 evaluable patients, 3 cardiac responses were observed. An additional patient had marked clinical improvement of heart failure. Four renal responses (out of 5 evaluable patients) and 2 hepatic responses (out of 2 evaluable) were also observed.
With a median follow-up of 13.0 months (m) from initiation of BLM therapy (range 1.1-33.8m), the median EFS was 22.3m and the median OS was 28.8m. At data cutoff 6/12 (50%) were still on therapy, 9/12 patients (75%) were alive, all 3 deaths were due to heart disease.
Seven patients (58%) had at least one grade 3 toxicity, included mostly infections/corneal toxicity. Apart from one event of grade 3 thrombocytopenia, there were no grade ≥3 hematological toxicities. No grade 4/5 toxicities were observed. Most patients (10/12, 83%) had corneal toxicity (6 patients- grade 1-2; 4 patients- grade 3), and dose reductions/delays were frequent (4/12 and 6/12, respectively). However, 7/10 patients with corneal toxicity improved overtime, no permanent visual disabilities were observed and only one patient stopped BLM due to keratopathy.
Discussion: While BLM provides an ORR of 30-45% in triple-refractory MM patients, it might have a greater efficacy in AL patients. The ORR in our heavily pre-treated AL patients, as well as in other studies (2 small, retrospective + preliminary results of the EMN27 prospective study (Kastritis, EHA conference 2023) approached 60-83%, and the EFS was also encouraging. This difference may be attributed to the lower burden of disease in AL compared to MM. Alternatively, AL plasma cells may be more sensitive to BLM, in a mechanism that still merits further exploration. Corneal toxicity was similar to what was seen in MM, and overall manageable. Hematological toxicity was significantly milder than that observed in MM, probably related to the better marrow reserve in AL.
Conclusions: BLM, being an off-the shelf immune therapy, with acceptable toxicity even in frail patients, can be a valuable option in AL, with a high ORR, and a signal for durable responses and high-quality organ responses in these patients.
OffLabel Disclosure:
Milani:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Siemens: Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria. Palladini:Prothena: Consultancy, Honoraria; Pfizer: Honoraria; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Argobio, GSK: Consultancy; Sebia: Honoraria; Siemens: Honoraria. Avivi Mazza:AbbVie: Honoraria.
Belantamab Mafodotin in Relapse/Refractory AL Amyloidosis

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal